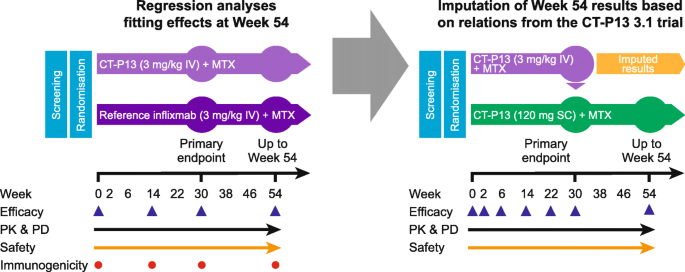
Comparative efficacy of subcutaneous (CT-P13) and intravenous infliximab in adult patients with rheumatoid arthritis: a network meta-regression of individual patient data from two randomised trials | Arthritis Research & Therapy | Full

PDF) Immunogenicity of the biosimilar CT-P13 infliximab or the original infliximab in Mohammed M. Kamil Iraqi patients with Ankylosing spondylitis does not correlate with their demographic characteristics Immunogenicity of the biosimilar CT-P13
Scientific rationale behind the development and approval of biosimilar infliximab (CT-P13) in Europe

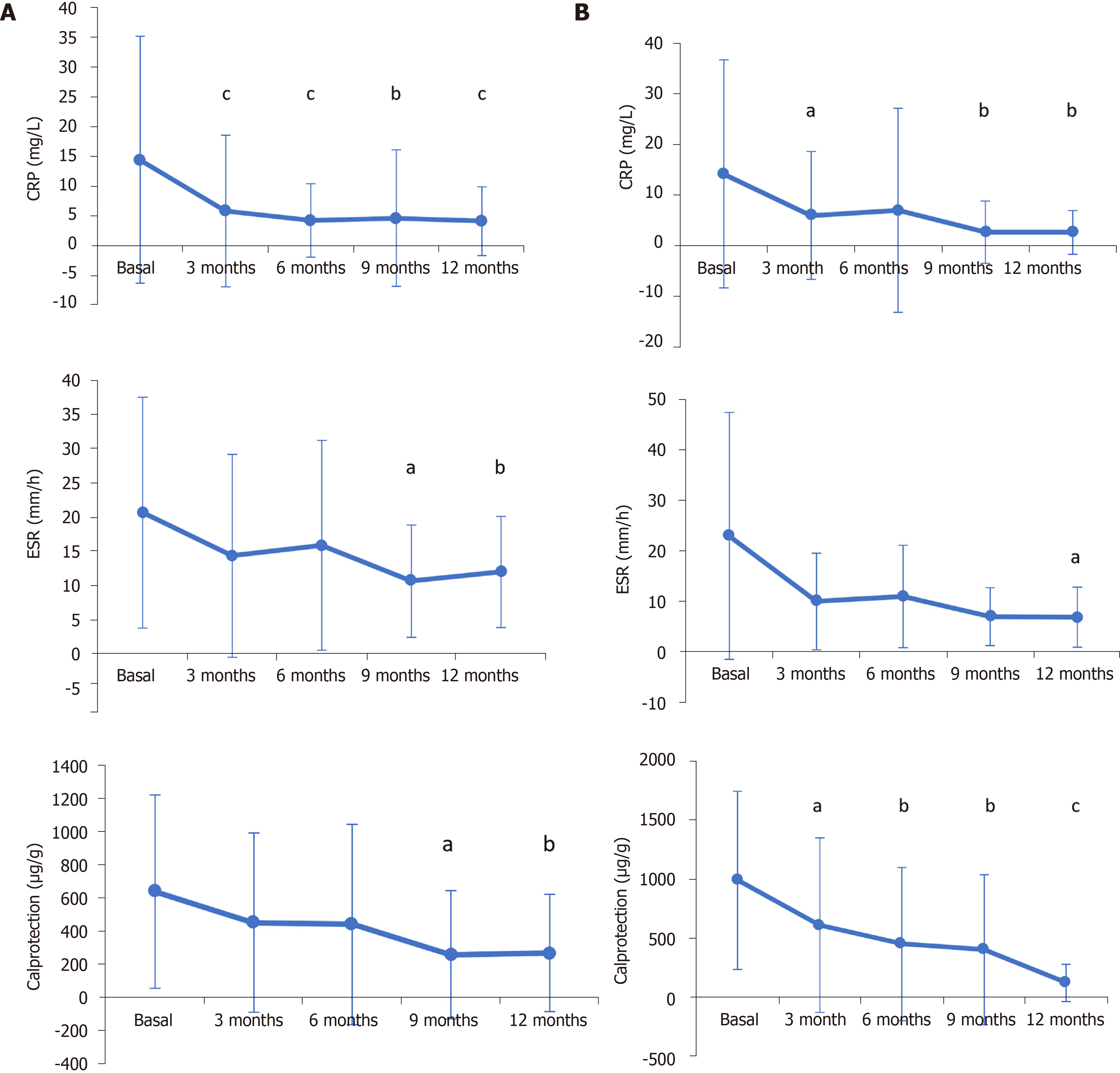
Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study | Annals of the

FRI0128 A NOVEL FORMULATION OF CT-P13 (INFLIXIMAB BIOSIMILAR) FOR SUBCUTANEOUS ADMINISTRATION: 1-YEAR RESULTS FROM A PART 1 OF PHASE I/III RANDOMIZED CONTROLLED TRIAL IN PATIENTS WITH ACTIVE RHEUMATOID ARTHRITIS | Annals of

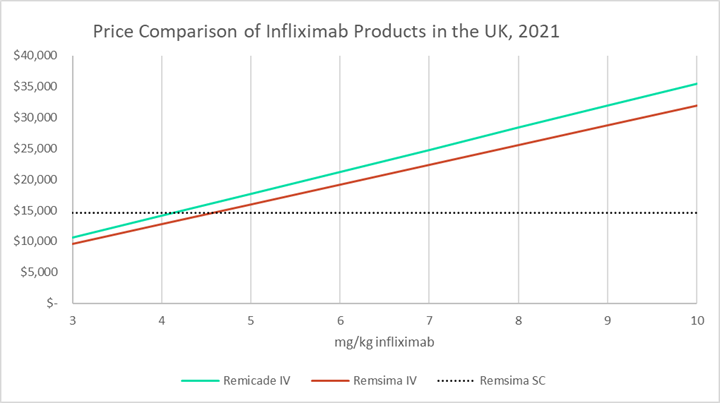
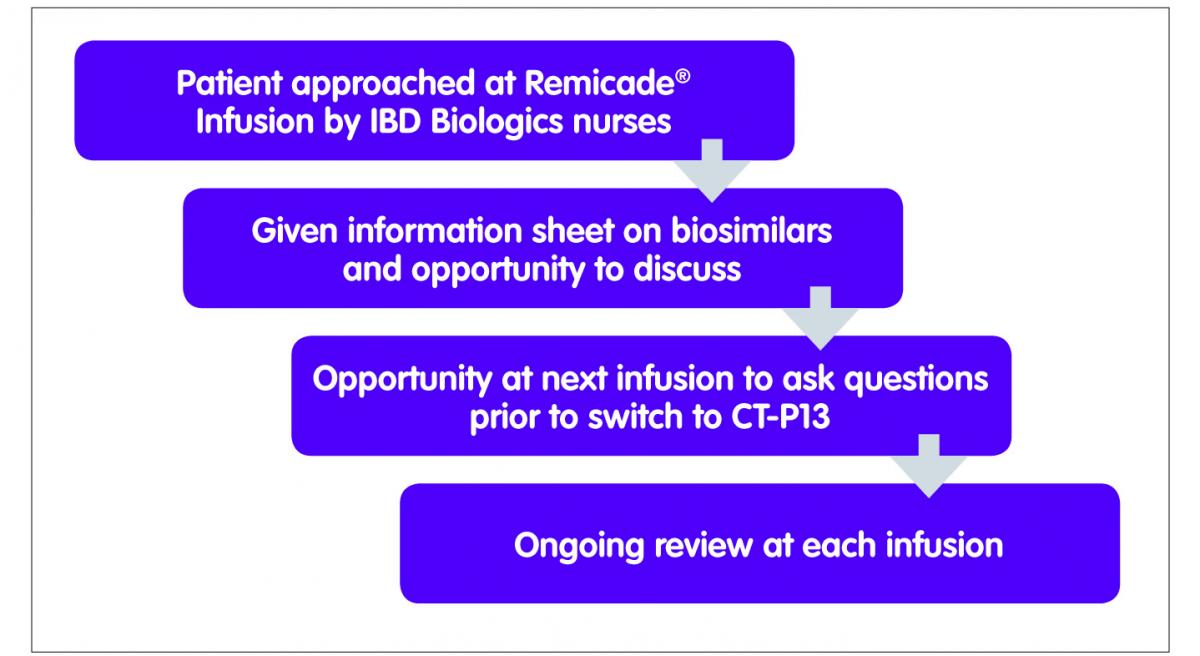
Biosimilar infliximab introduction into the gastroenterology care pathway in a large acute Irish teaching hospital: a story behind the evidence - GaBI Journal

PDF) Long‐term efficacy and safety of biosimilar infliximab (CT‐P13) after switching from originator infliximab: Open‐label extension of the NOR‐SWITCH trial

PDF) Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: Comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study
Analysis of clinical trials of biosimilar infliximab (CT-P13) and comparison against historical clinical studies with the inflix

PDF) Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: Comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study

Celltrion USA announces submission of the Biologics License Application (BLA) of novel subcutaneous formulation of CT-P13 to U.S. Food and Drug Administration | Business Wire

Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn's disease: an international, randomised, double-blind, phase 3 non-inferiority study - The Lancet
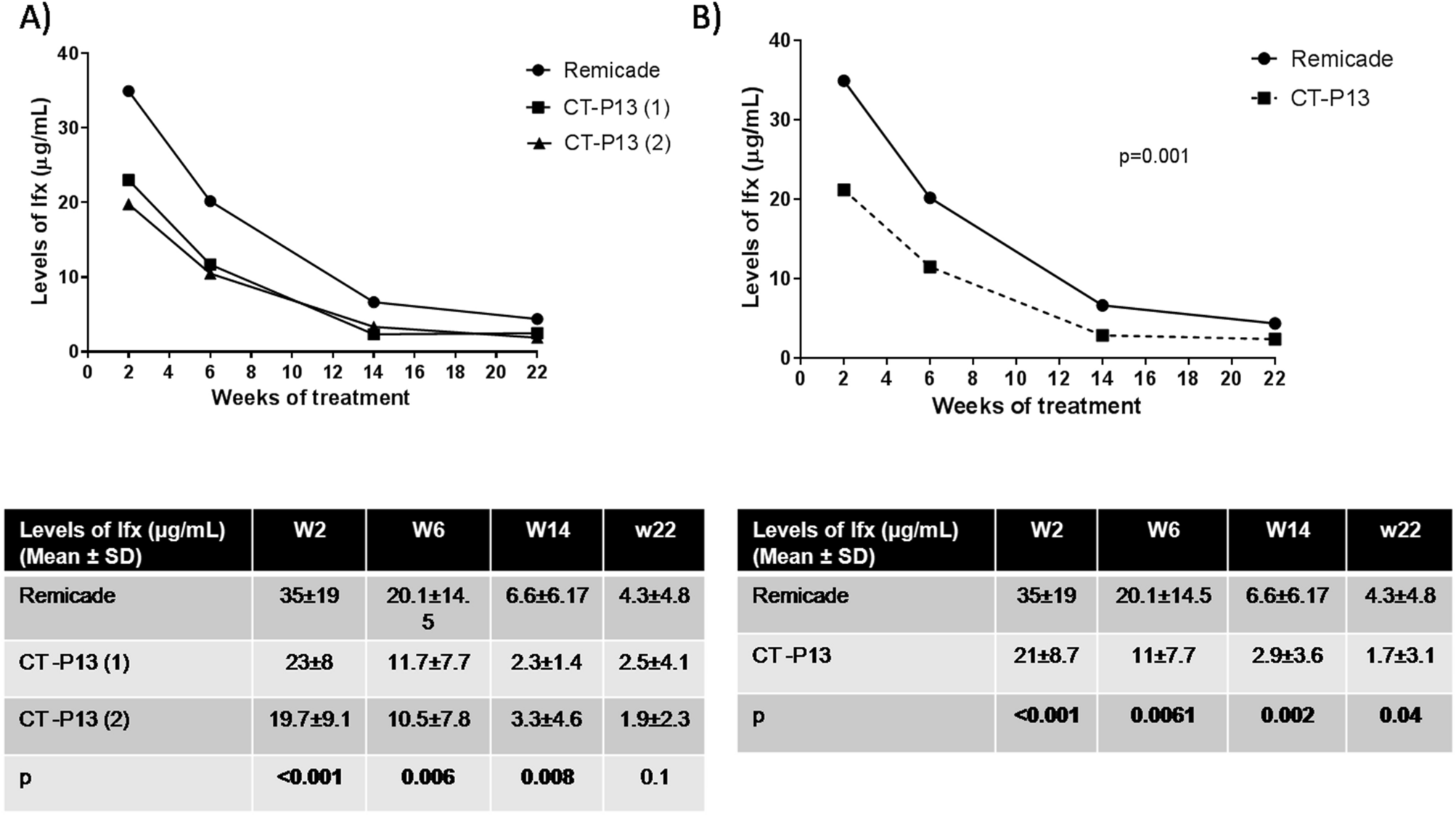
Infliximab concentrations in two non-switching cohorts of patients with inflammatory bowel disease: originator vs. biosimilar | Scientific Reports
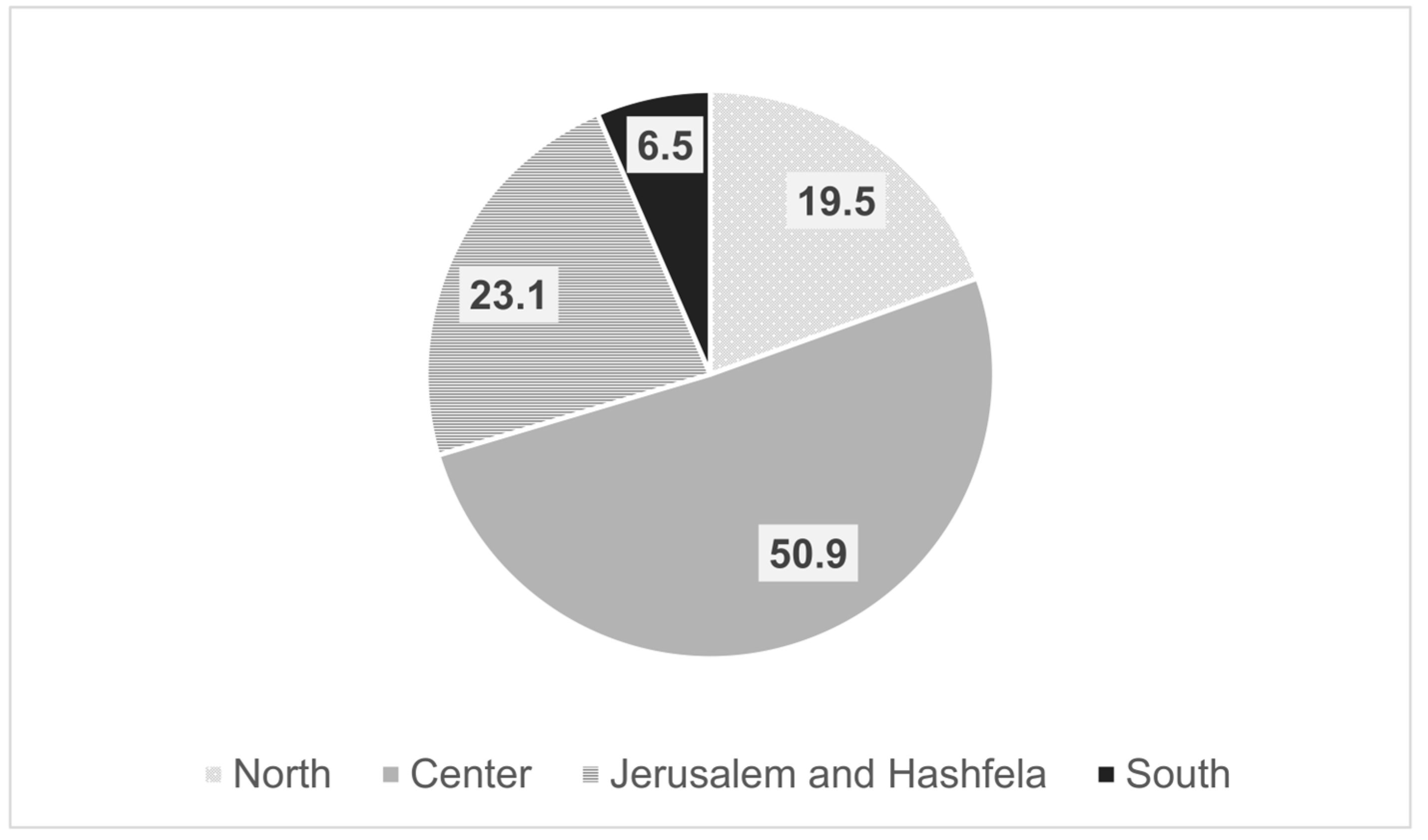
Medicina | Free Full-Text | The Perception among Israeli Gastroenterologists Regarding Treatment of Patients with Biosimilar Medications

Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease - ScienceDirect
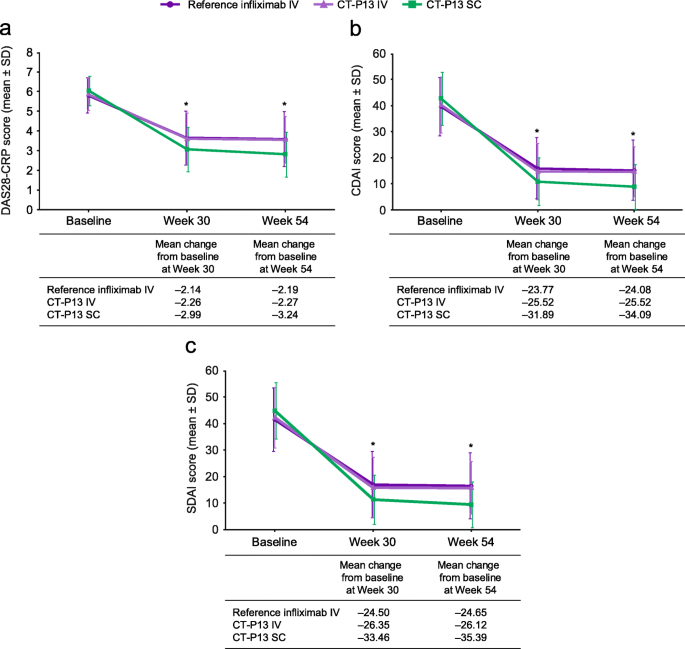
Comparative efficacy of subcutaneous (CT-P13) and intravenous infliximab in adult patients with rheumatoid arthritis: a network meta-regression of individual patient data from two randomised trials | Arthritis Research & Therapy | Full