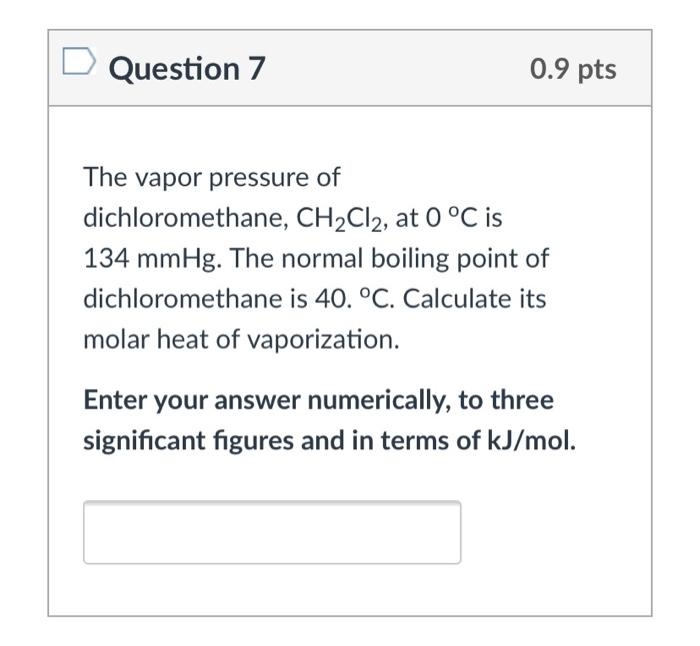
Free Online Help: The vapor pressure of dichloromethane CH2Cl2 at 0`C in 134 mm Hg . The normal boiling point of dichloromethane is 40 `C . Calculate its molar heat of vapourization

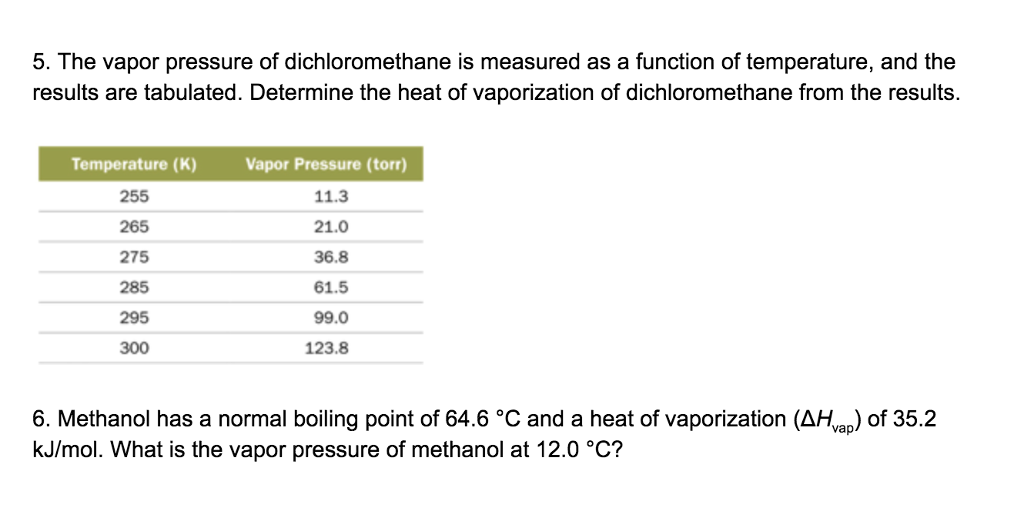
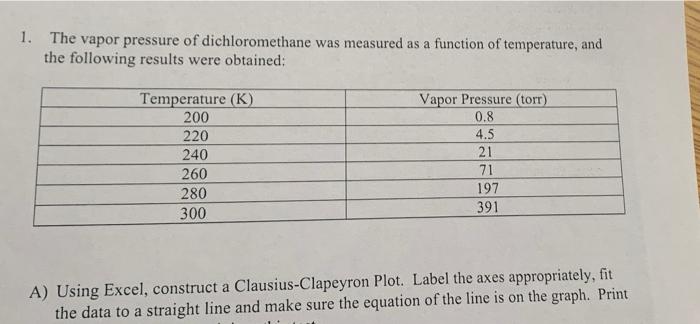
SOLVED: The vapor pressure of dichloromethane was measured as a function of temperature, and the following results were obtained: Determine: (12 points) a. the heat of vaporization of dichloromethane. b. The temperature

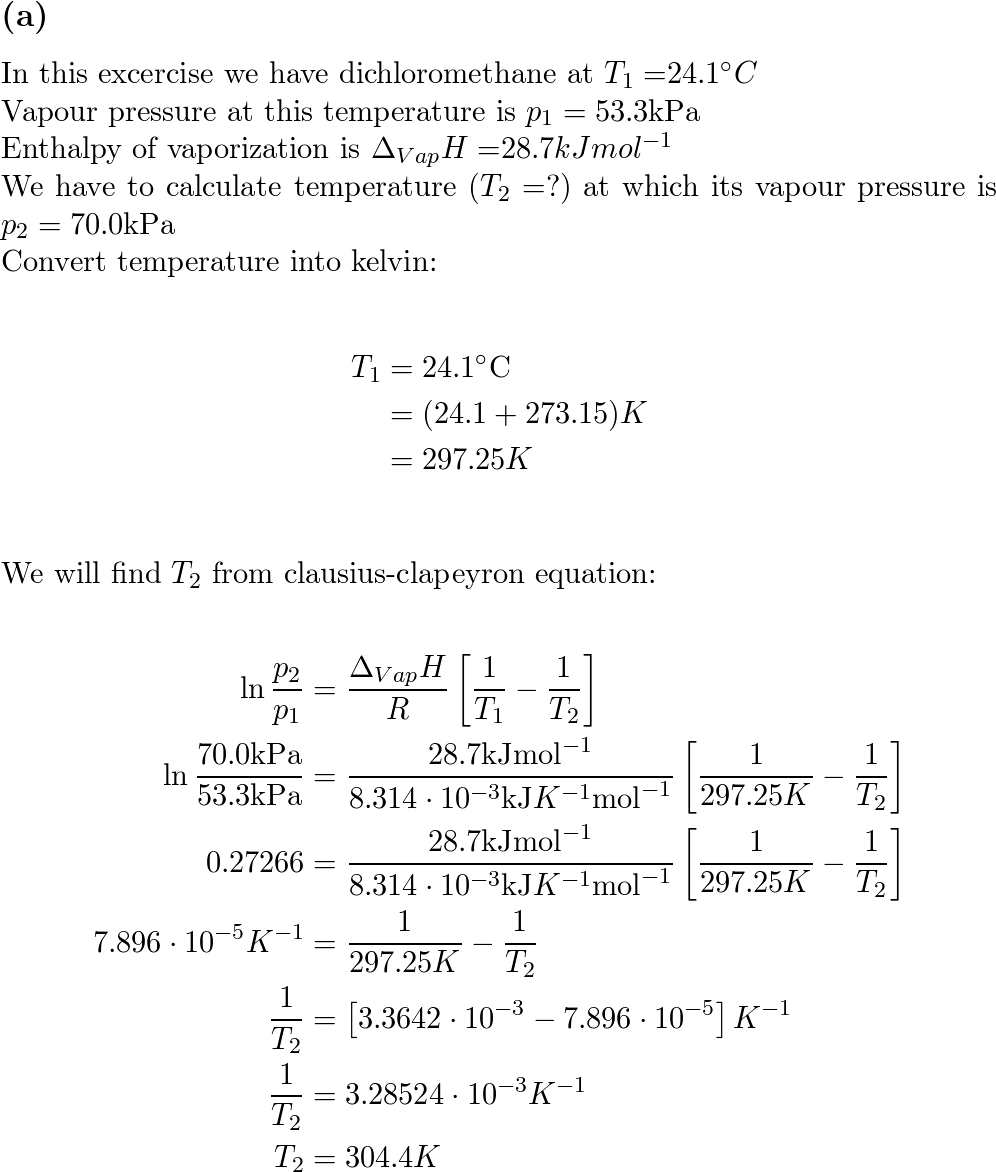
SOLVED: Question 1 The vapour pressure of dichloromethane at 24.1oC is 400 Torr and its enthalpy of vaporization is 28.7 KJ mol-1. Estimate the temperature at which its vapour is 500 Torr.

Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25oC are 200 mmHg and 41.5 mmHg respectively, Vapour pressure of the solution obtained by mixing 25.5 g of CHCl3 and 40 g

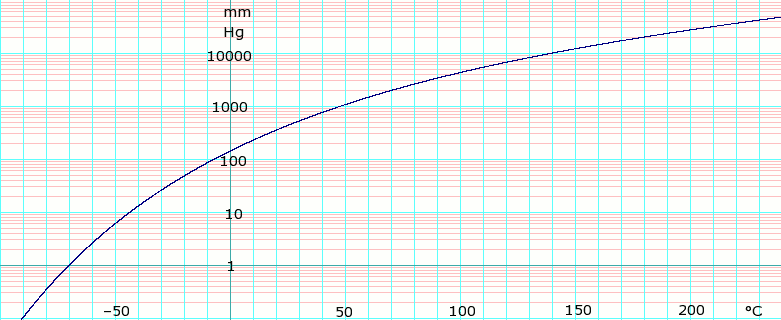
SOLVED: The normal boiling point of dichloromethane is 39.6 *C and its enthalpy of vaporization Is 28.1 kJlmol, What is the vapor pressure of dichloromethane at 15 "C? 661 mm Hg 302

Isobaric Vapor–Liquid Phase Equilibrium Measurements of the Dichloromethane–Titanium Tetrachloride System at 101,325 Pa | SpringerLink

The vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 298 K is 200 mm Hg and 415 mm Hg, respectively. If a solution is prepared by mixing 25.5 g of CHCl3

Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 298 K are 200 mm Hg and 415 mm Hg respectively. Calculate the vapour pressure of the solution prepared by mixing 25.5 g

Equilibrium concentration of dichloromethane vapor as a function of... | Download Scientific Diagram

Fig. The isotherms of dichloromethane (DCM) vapours sorption (a), the... | Download Scientific Diagram