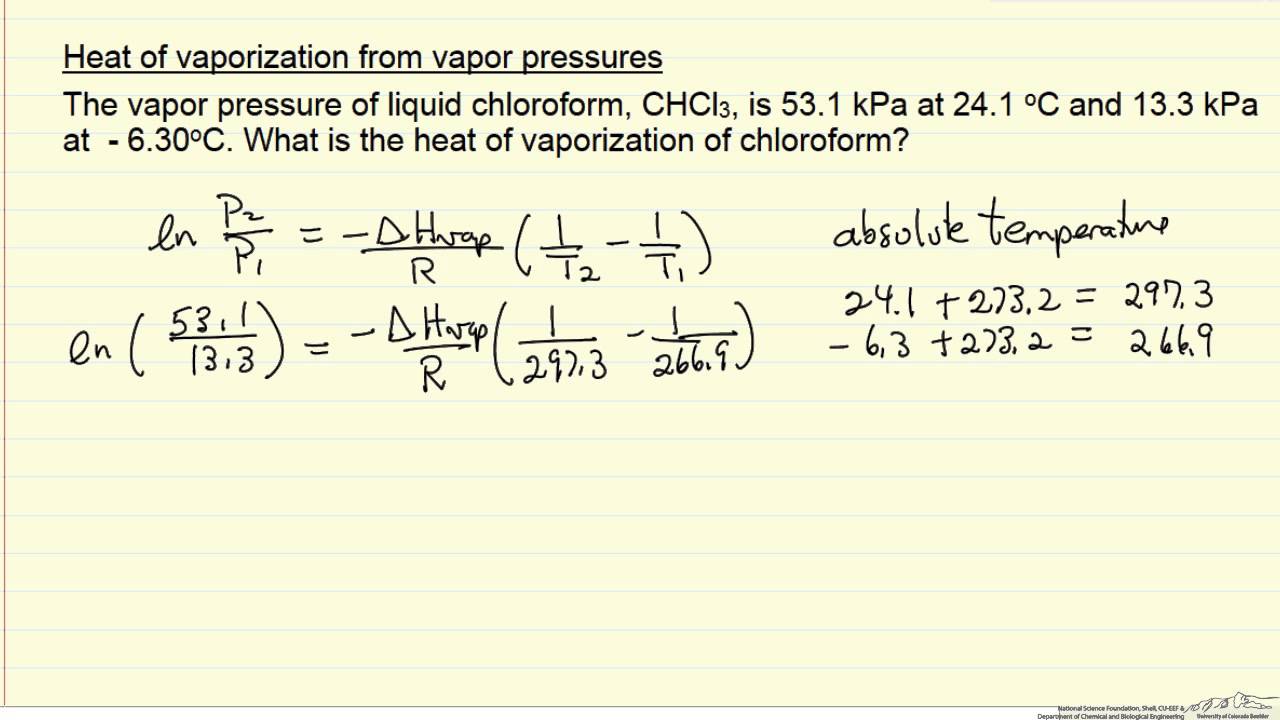
Enthalpy of vaporization vs. temperature for the truncated and shifted... | Download Scientific Diagram

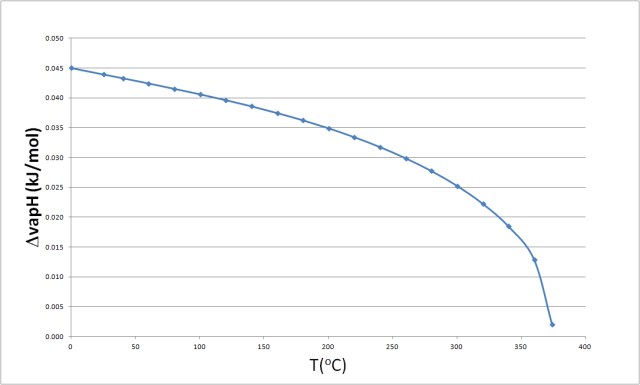
Enthalpy of vaporization of water: (—) Reference fundamental equation... | Download Scientific Diagram

enthalpy - What is heat of vaporization? How can it be used at temperature as low as 25 °C? - Chemistry Stack Exchange

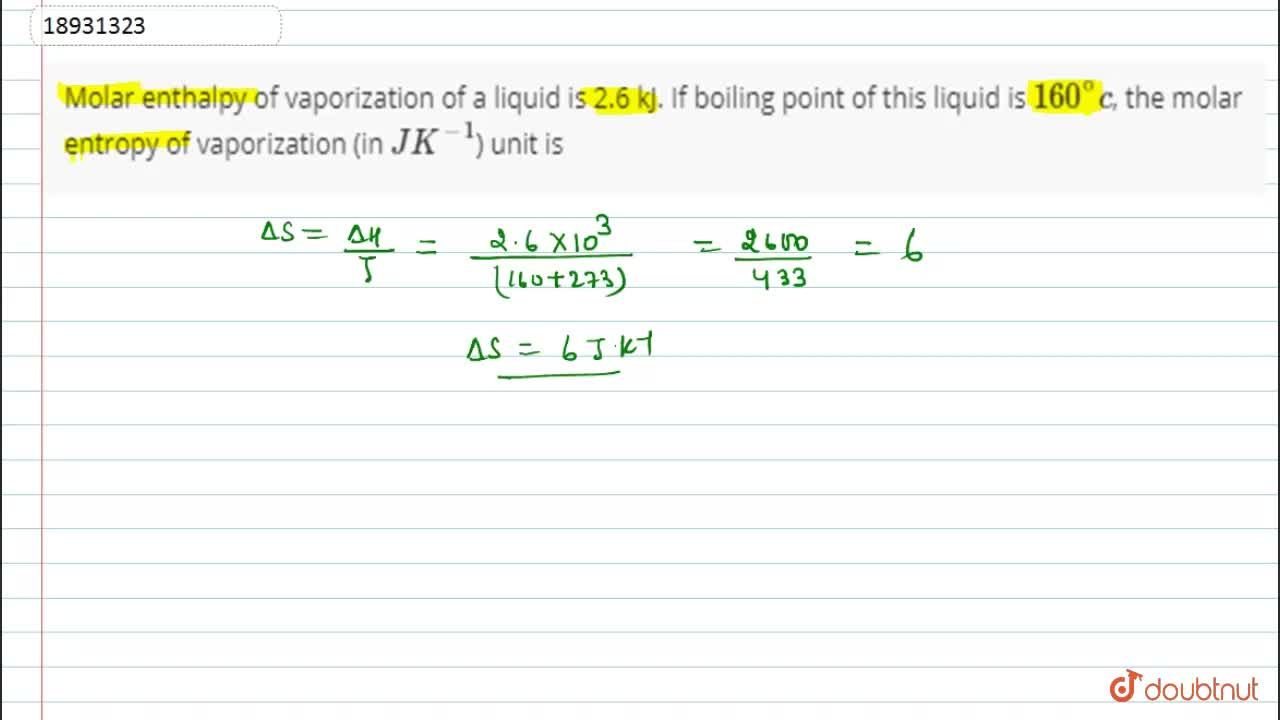
SOLVED: Heat of Vaporization 28 Calculate the molar heat of vaporization of water using the graph YOu generated from Excel. Show the calculations below. F Siope Alvie 5311.5 J 16uuJ Slopq 6388,2




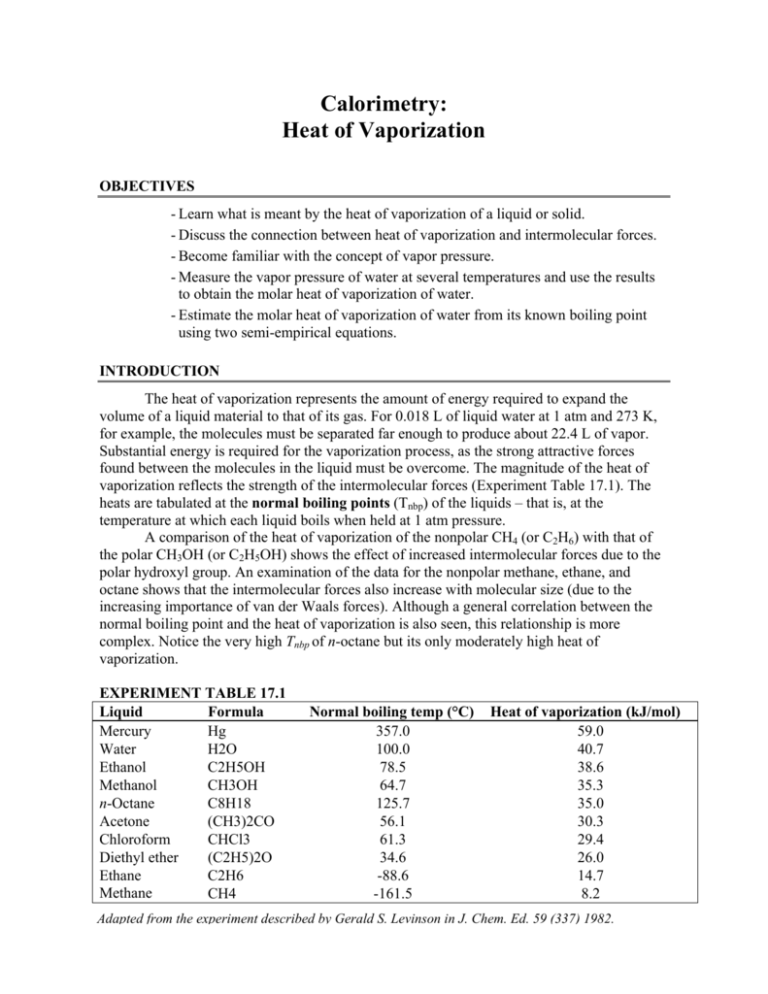


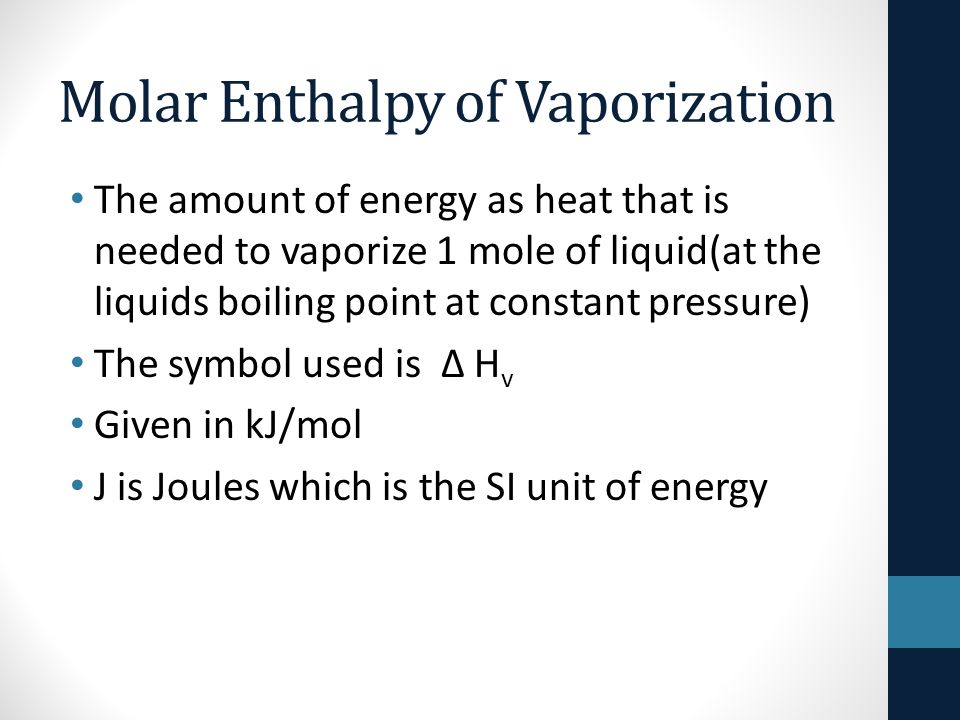

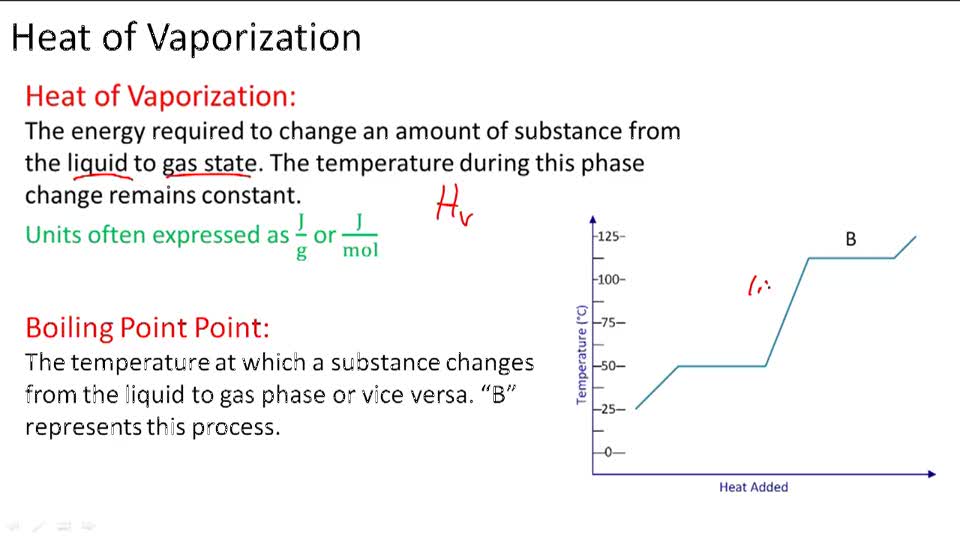
![PDF] Prediction of the enthalpy of vaporization of metals and metalloids | Semantic Scholar PDF] Prediction of the enthalpy of vaporization of metals and metalloids | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d72020d4fcbf01ad742396cd253e8efacc480043/4-Table2-1.png)