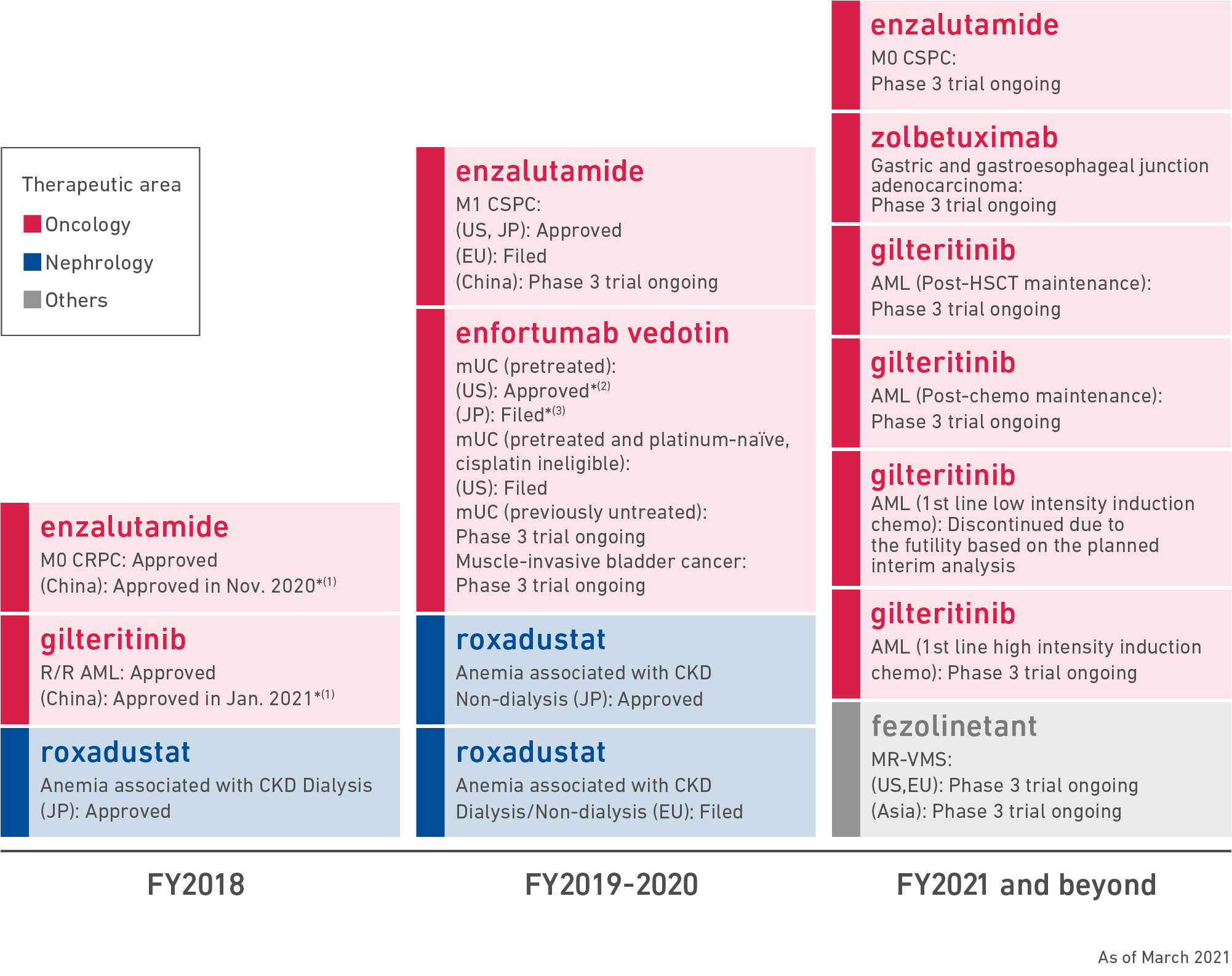
Building a foundation to create innovative treatments under the Corporate Strategic Plan 2018 | Astellas Pharma Inc.

PDF) A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause
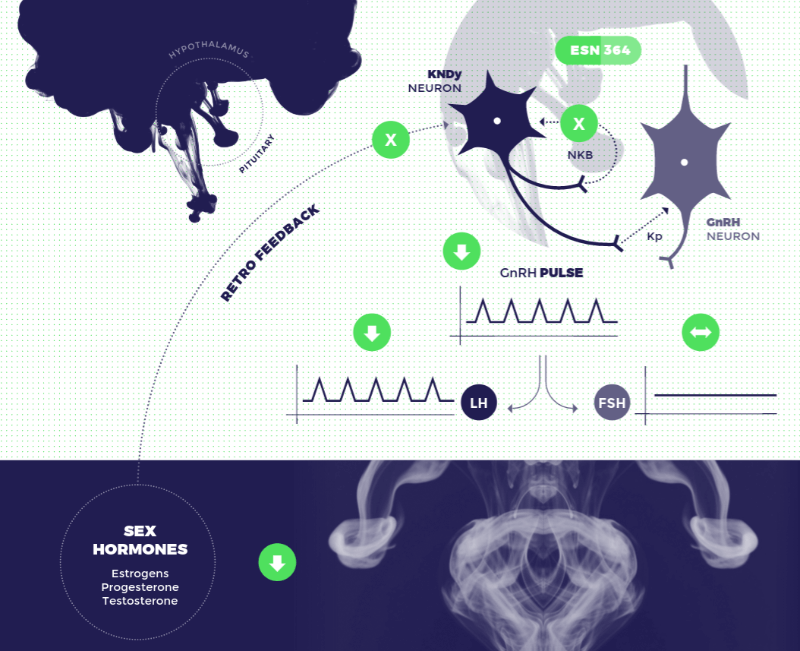
New therapeutics options for Vasomotor Symptoms of Menopause are becoming available as 9+ companies are working on drug profiles | MENAFN.COM

Fezolinetant (ESN364): Astellas' Neurokinin 3 Receptor Antagonists Under Development for the Treatment of Vasomotor Symptoms - Global Emerging Insight and Market Forecast to 2030 - ResearchAndMarkets.com | Business Wire