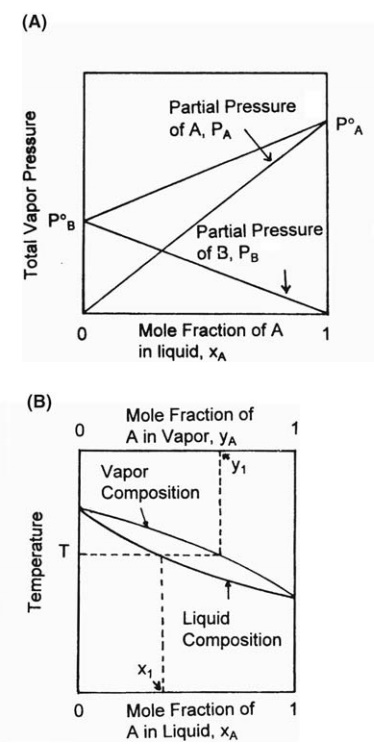
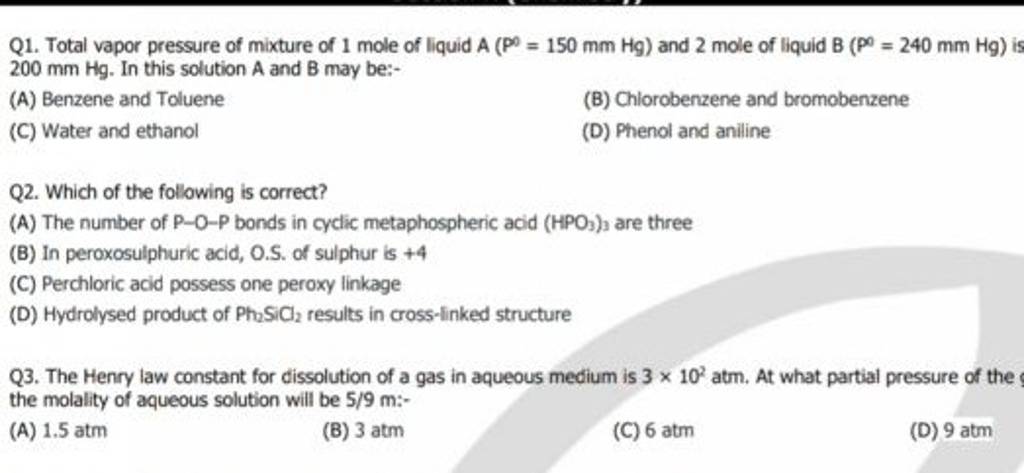
Total Vapour pressure of mixture of 1molA`(p_(A)^(0)=150 \"torr\")`and 2molB `(p_(B)^(0)=240 \"to - YouTube

physical chemistry - What causes the lowering of vapour pressure in volatile/nonvolatile solvent mixtures? - Chemistry Stack Exchange

Vapor Pressures and Thermophysical Properties of Dimethoxymethane, 1,2-Dimethoxyethane, 2-Methoxyethanol, and 2-Ethoxyethanol: Data Reconciliation and Perturbed-Chain Statistical Associating Fluid Theory Modeling | Journal of Chemical & Engineering Data

Saturated vapor pressures of CEC gasoline (measured), the 3C mixture... | Download Scientific Diagram

The total vapour pressure, Ptotal (in torr) for a mixture of two volatile components, A and B is given by Ptotal = 220 - 110 XB Where, XB is mole fraction of

CHEM 201 - Finding mole fraction from vapor pressure of a mixture with two volatile liquids - YouTube
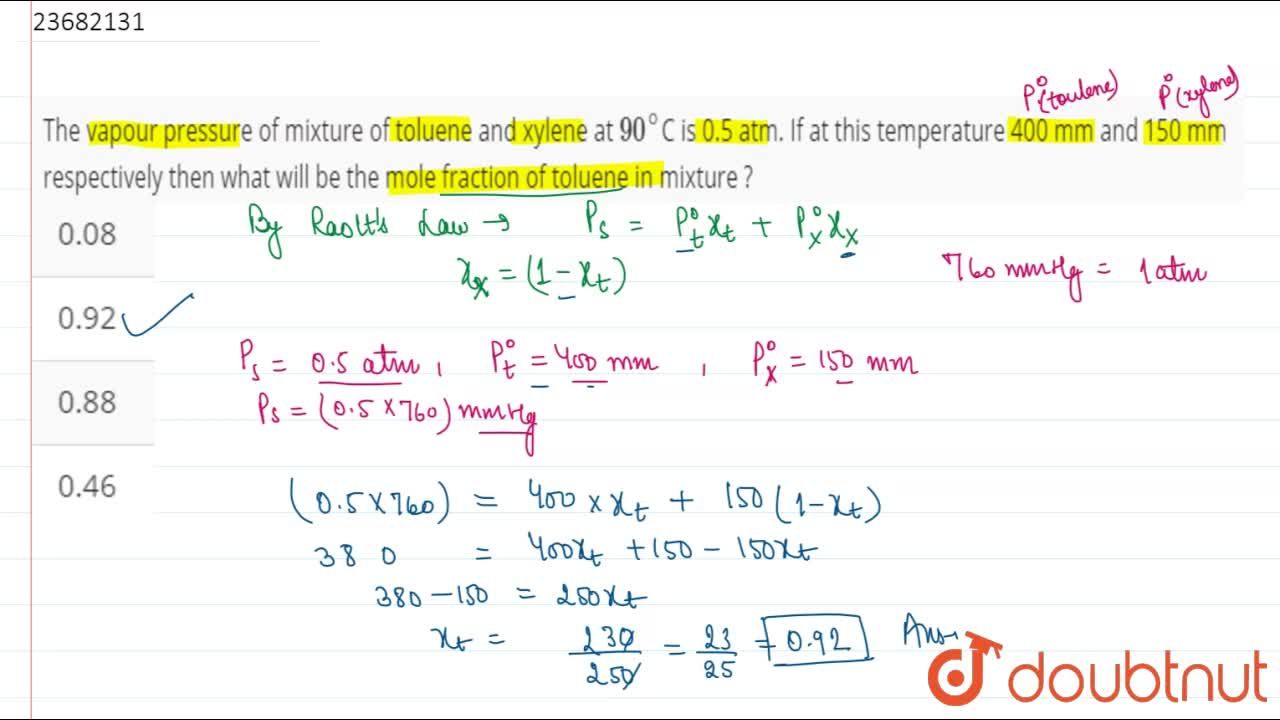
The vapour pressure of mixture of toluene and xylene at 90^(@)C is 0.5 atm. If at this temperature 400 mm and 150 mm respectively then what will be the mole fraction of



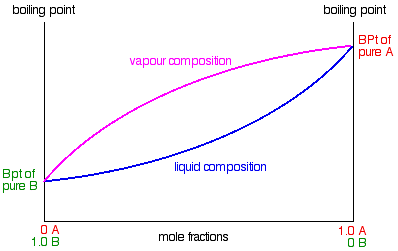



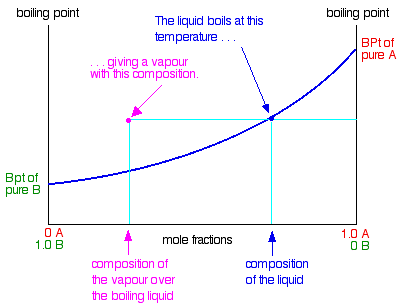

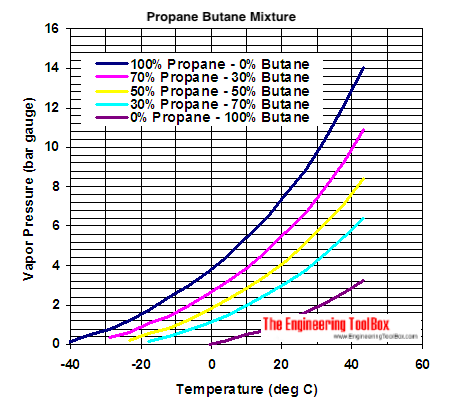
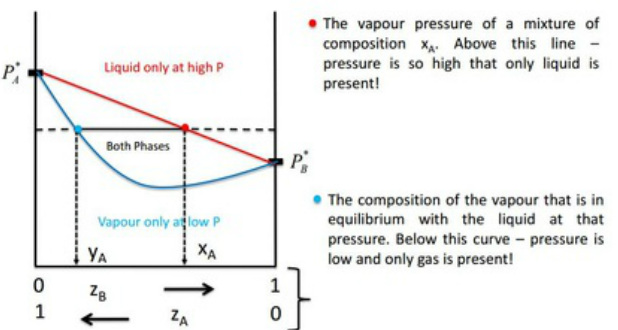

:max_bytes(150000):strip_icc()/GettyImages-1125699119-5c59ca0846e0fb000152fc04.jpg)